How Does Calcium Obey the Octet Rule When Reacting to Form Compounds
6.10: The Octet Rule
-
- Last updated
- Save as PDF
- Page ID
- 49354
-

- ChemPRIME at Chemical Education Digital Library (ChemEd DL)
Because binary ionic compounds are confined mainly to group 1 and group 2 elements on the one hand and group VI and VII elements on the other, we find that they consist mainly of ions having an electronic structure which is the same as that of a noble gas. In calcium fluoride, for example, the calcium atom has lost two electrons in order to achieve the electronic structure of argon, and thus has a charge of +2:
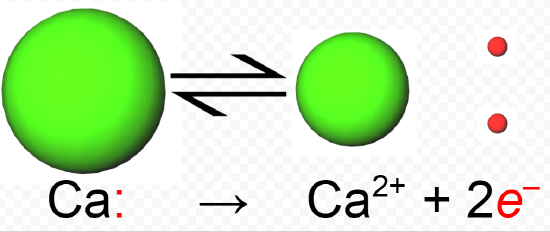
1s 22s 22p 63s 23p 6 4s 2 → 1s 22s 22p 63s 23p 6 + 2e –
By contrast, a fluorine atom needs to acquire but one electron in order to achieve a neon structure. The resulting fluoride ion has a charge of –1:
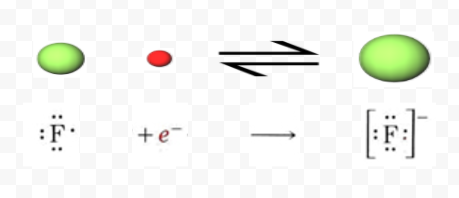
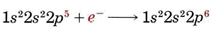
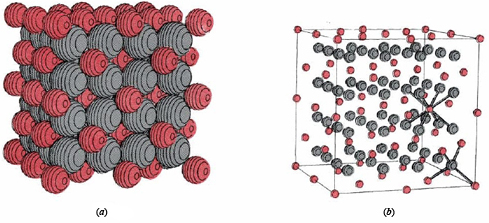
The outermost shell of each of these ions has the electron configuration ns 2 np 6, where n is 3 for Ca2+ and 2 for F–. Such an ns 2 np 6 noble-gas electron configuration is encountered quite often. It is called an octet because it contains eight electrons. In a crystal of calcium fluoride, the Ca2+ and F– ions are packed together in the lattice shown below. Careful study of the diagram shows that each F– ion is surrounded by four Ca2+ ions, while each Ca2+ ion has eight F– ions as nearest neighbors.
Thus there must be twice as many F– ions as Ca2+ ions in the entire crystal lattice. Only a small portion of the lattice is shown, but if it were extended indefinitely in all directions, you could verify the ratio of two F– for every Ca2+. This ratio makes sense if you consider that two F– ions (each with a –1 charge) are needed to balance the +2 charge of each Ca2+ ion, making the net charge on the crystal zero. The formula for calcium fluoride is thus CaF2.
Newcomers to chemistry often have difficulty in deciding what the formula of an ionic compound will be. A convenient method for doing this is to regard the compound as being formed from its atoms and to use Lewis diagrams. The octet rule can then be applied. Each atom must lose or gain electrons in order to achieve an octet. Furthermore, all electrons lost by one kind of atom must be gained by the other.
An exception to the octet rule occurs in the case of the three ions having the He 1s 2 structure, that is, H–, Li+ and Be2+. In these cases two rather than eight electrons are needed in the outermost shell to comply with the rule.
Example \(\PageIndex{1}\): Ionic Formula
Find the formula of the ionic compound formed from O and Al.
Solution
We first write down Lewis diagrams for each atom involved:
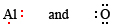
We now see that each O atom needs 2 electrons to make up an octet, while each Al atom has 3 electrons to donate. In order that the same number of electrons would be donated as accepted, we need 2 Al atoms (2 × 3e – donated) and 3 O atoms (3 × 2e – accepted). The whole process is then
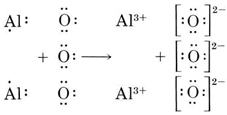
The resultant oxide consists of aluminum ions, Al3+, and oxide ions, O2–, in the ratio of 2:3. The formula is Al2O3.
How Does Calcium Obey the Octet Rule When Reacting to Form Compounds
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Book:_ChemPRIME_%28Moore_et_al.%29/06:_Chemical_Bonding_-_Electron_Pairs_and_Octets/6.10:_The_Octet_Rule